Separating amid confirmation and approval for an assembling procedure can be befuddling, as they are the same. The distinction is in usage, wanted result and period of the procedure. To endeavor to clear things up, take a glance at a basic case of a rigging gear-tooth that has been produced according to prerequisites expressed by a customer – The initial step is to contrast machine gear-piece with outline prerequisites of material, measurements, resistance, and so on. Now, you are not concentrating on whether it will work in the machine it is planned for, but instead, whether gear-tooth is produced inside parameters showed or not. This is confirmation, which is a demonstration of testing and affirming that the item meets particular necessities of the characterized guidelines.
The way that the machine gear-piece meets prerequisites according to plan isn’t an assurance that it will work, as guidelines might miss some data that is basic to its execution. The following stage is to gather it in the machine intended for and test it through different tasks to ensure it fills in. This is approval, the demonstration of testing and affirming that the item can play out its proposed reason and create worthy outcomes. It is only a basic cog apparatus, however building mind-boggling hardware, the probability of unexpected obstacles increments exponentially. Both check and approval assume basic parts in quality administration, lessening blunders and ensuring item complies with client’s prerequisites, industry measures, and administrative expert rules.
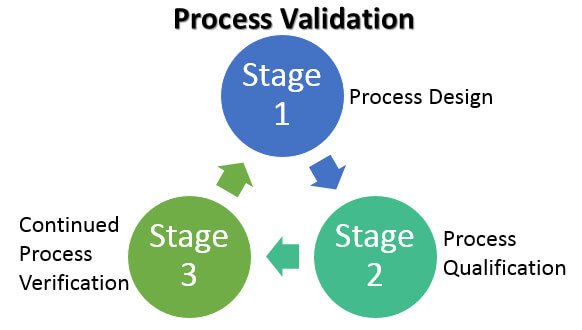
Process Validation Lifecycle Image source: emersonautomationexperts.com
When will you require Process Verification?
Confirmation assumes a part in relatively every stage, from introductory improvement to creation and upscaling. Approval becomes the important factor later in assembling lifecycle once the item is checked and tried. A portion of confirmation forms in each period of assembling are –
Advancement:
When underlying plans are being drawn up, singular segments are displayed and the similarity of a considerable number of parts is checked. The individual parts and also item overall ought to have the capacity to exhibit for creating results inside the client’s predetermined prerequisites.
Survey:
A model of the whole framework is made and checked for adherence to the prerequisites. Adjustment to different confinements is checked; the outline is explored and modified as required.
Creation:
Throughout the generation stage, items ought to be subjected to standard confirmation tests to guarantee steady quality. Remedial and preventive activities, establishment capability and operational capability are types of check that are completed amid generation.
Scale up:
When an item is prepared in bigger volumes, a check is as yet a vital angle for guaranteeing every item that is discharged in the market will create worthy outcomes. This should be possible as irregular examinations for quality and different checks.
When will you need Process Validation?
Approval starts amid the generation stage, can be executed close by check and plays an extensive variety of parts in making a palatable item. It can be utilized as a part of the place of a check. This is more often than not in circumstances in which outcomes can’t be estimated, or cost of checking is too high to legitimize the requirement for confirmation. Prior to scaling up generation, executed approval in various test conditions guarantees worthy execution when the item is utilized as a part of true situations. Indeed, even with strict procedures set up to guarantee item quality and powerful counteractive action instruments, surprising outcomes can at present happen. The validation can distinguish and remedy these blunders showcase discharge. Execution capability requires stringent approval checks to ensure the item can perform to level it is intended for.
FirstSource Laboratory Solutions LLP has dedicated Service Engineer’s team, who gives full support from installation of the product till verified/validated the guidelines.
Completely Verified or Validated?
Confirmation can more often than not be utilized to control process yields. However, there are a few situations in which check may have restricted utility or even be inconceivable if the yield can’t be estimated. According to quality systems regulations, forms that influence the critical to quality can be approved with a high level of confirmation and affirmed by built-up methodology. That is to state, determine that deformities emerging from unconfirmed procedures are far-fetched. The procedure itself can be approved and varieties can be controlled by utilizing reasonable strategies like quality by design. The procedure approval should be supported and joined by suitable documentation. To decide whether a procedure requires approval or confirmation, numerous variables need to be studied attentively. These incorporate the adequacy and exactness of confirmation, dangers identified with the procedure and the practicality of routine tests.
The probability of deformities can be decreased by completely understanding the prerequisites. Asking for extra subtle elements for factors and checking at each point in the lifecycle. In any case, sometimes there are still glitches and that is the reason approval can be justified even despite the venture.
 FirstSource Laboratory Solutions Official Blog First Indian Scientific Online Shop
FirstSource Laboratory Solutions Official Blog First Indian Scientific Online Shop